Last month, I outlined the four pillars of the current longevity stack. While that framework is useful for categorization, the real value lies in how these components integrate to solve specific clinical bottlenecks.
Today, I want to look at a specific case study: cardiovascular health. The field is moving from a model of simple lipid management toward “debugging” the underlying biology and architecting the biomimetic repair infrastructure required for true longevity.
The starting point is a major review published in Nature Reviews Cardiology (2026), which identifies a critical challenge: “Residual Cardiovascular Risk.”
The paper highlights a critical nuance. While aggressive lipid management has been a triumph of modern medicine, it has reached a point of diminishing returns. Even with optimal care, patients face a persistent risk driven by other factors, specifically, inflammatory pathways.
This clinical finding connects directly to Pillar 1 (Foundational Science). If inflammation is the signal identifying this residual risk, the next logical step is to understand the biological mechanism generating it. One area where that foundational science is moving forward at a steady pace is the study of cellular senescence.
What is Senescence?
In the context of biological aging, cellular senescence is a state where damaged cells stop dividing but refuse to die. Instead of being cleared by the immune system, they linger in the tissue as “zombie” nodes.
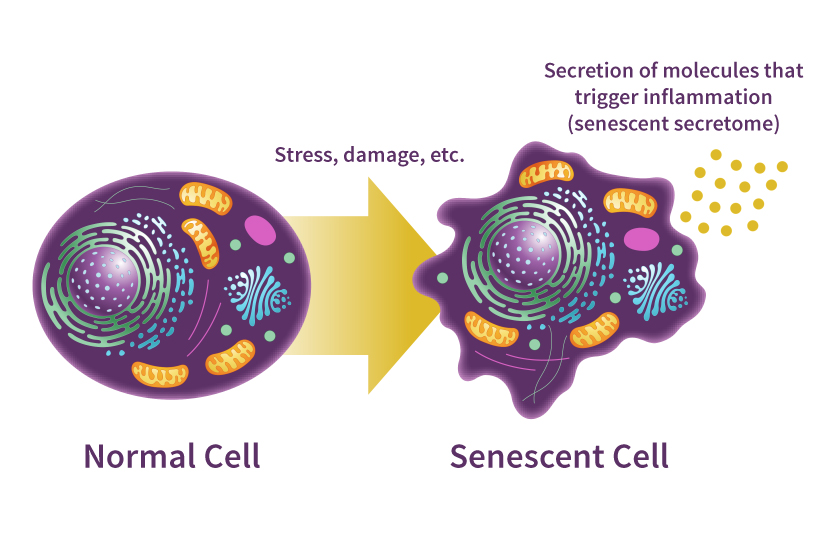 Diagram illustrating the transition from healthy cells to senescent ‘zombie’ cells and their impact on surrounding tissue. Source: National Institute on Aging (NIH)
Diagram illustrating the transition from healthy cells to senescent ‘zombie’ cells and their impact on surrounding tissue. Source: National Institute on Aging (NIH)
While this mechanism is a critical tumor-suppressor in early life, its accumulation in aged tissue becomes a primary driver of decay. This is a classic example of antagonistic pleiotropy (Campisi, 2005), the principle where a mechanism that is beneficial in early life becomes detrimental as the organism ages.
This “zombie cell” accumulation is a systemic challenge, impacting tissues from the lungs to the kidneys. While I am focusing on the cardiovascular implications today, the broader impact of senescence across the four pillars is something I will explore in future posts.
Within Pillar 1, researchers are moving past identification toward active intervention. There is steady progress in the ability to detect and address these drivers of decline. This transition from decoding biology to engineering repair defines the next era of longevity.
Managing Residual Risk
The practice of medicine has seen progress in managing the most visible drivers of cardiovascular decay. Over the last few decades, Major Adverse Cardiovascular Events (MACE) have come down, driven largely by the widespread adoption of statins and, more recently, modern PCSK9 inhibitors.
A recent meta-analysis of eleven clinical trials involving over 135,000 patients led by Dr. Alberto Cordero (Cordero et al., 2023) established that intensive lipid-lowering therapies reduce the risk of MACE by an average of 15% and cardiovascular mortality by 6%. Building on this, clinical trials such as FOURIER (Sabatine et al., 2017) and ODYSSEY Outcomes (Schwartz et al., 2018) have shown that modern PCSK9 inhibitors can reduce LDL cholesterol by 50-70% and further lower the risk of MACE by an additional 15-20% in high-risk populations.

Researchers suggest the answer to this stagnation lies in “residual inflammatory risk.” As established in The Lancet (2023), inflammation, specifically as measured by high-sensitivity C-reactive protein (hsCRP), can be a stronger predictor of future cardiovascular events and all-cause mortality than LDL cholesterol levels for patients already receiving statin therapy.
This suggests that the next frontier is not about managing lipids, but addressing the aging of the vascular tissue itself.
The SASP: A Biological Broadcast Storm
A major driver behind this elevated hsCRP signal is the Senescence-Associated Secretory Phenotype (SASP).
To understand the SASP, it is helpful to use a universal engineering analogy: a broadcast storm. Imagine a malfunctioning Ethernet node or a “stuck mic” on a radio frequency. These senescent cells don’t simply stop functioning. They begin broadcasting a constant stream of pro-inflammatory signals, including cytokines like IL-6, which triggers the liver to produce CRP, chemokines, and matrix metalloproteinases (MMPs). This noise eventually drowns out healthy cellular intent, polluting the environment and degrading the structural integrity of the heart and arteries. This is a core driver of myocardial fibrosis and arterial stiffening.
Reconstructing the Biological Infrastructure
Clearing the broadcast storm of the SASP via senolytics is a necessary first step, but it is not enough for longevity improvements. Removing the malfunctioning nodes stops the active signal pollution, but it does not automatically resolve the accumulated structural debt, which is the stiff, non-conductive scar tissue left behind by years of chronic inflammation.
Moving from cleanup to active repair requires a solution for the lack of organized cellular housing. Professor Onnik Agbulut and his team at Sorbonne University’s Institute of Biology Paris-Seine are addressing this by engineering bio-inspired biomaterials. These synthetic structures, including 3D scaffolds and electrospun nanofibers, function as a surrogate for the extracellular matrix. By providing this environment (Kitsara, Agbulut, et al., 2023), the Sorbonne team is creating the structural template required for new, healthy cells, such as human induced pluripotent stem cell-derived cardiomyocytes, to colonize and restore functional capacity to the heart.
The Roadmap Forward
The focus in cardiovascular health is shifting from simple mitigation to a roadmap of active biological repair. From a technologist’s perspective, the critical path to making these therapies a reality is the implementation reality of clinical delivery. The challenge is moving from successful in-vitro scaffolding to safe, scalable human applications.
The roadmap for these advances looks like this:
- Quieting the Noise: Validating targeted senolytic interventions that can resolve the inflammatory burden of the SASP without systemic toxicity.
- Repair Infrastructure Deployment: Engineering the delivery mechanisms for biomimetic scaffolds to ensure they can host and mature functional cardiomyocytes within a living heart.
By addressing the residual inflammatory risk and structural debt at their source, the next generation of therapeutics will move beyond patching a legacy architecture. This transition from “managing decline” to “architecting repair” is the shift required to move the needle on human longevity. The field is learning to reinforce biological integrity at the cellular level.
References
- Campisi, J. (2005). Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell, 120(4), 513-522.
- Cordero, A., et al. (2023). The efficacy of intensive lipid-lowering therapies on the reduction of LDLc and of major cardiovascular events. Journal of Clinical Lipidology, 17(4), Supplement, S1-S2.
- Sabatine, M. S., et al. (2017). Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine, 376(18), 1713-1722.
- Schwartz, G. G., et al. (2018). Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. New England Journal of Medicine, 379(22), 2097-2107.
- Ridker, P.M., et al. (2023). Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. The Lancet.
- Ottaviani, A., et al. (2024). Mechanisms of Telomere-Driven Senescence and Immune Evasion. IRCAN Research Perspectives.
- Kitsara, M., Kontziampasis, D., Agbulut, O., & Chen, Y. (2023). Surfaces for hearts: Establishing the optimum plasma surface engineering methodology on polystyrene for cardiac cell engineering. Applied Surface Science, 620, 156822.
- Batoumeni, V., Agbulut, O., et al. (2025). Integrated phenotypic and transcriptomic characterization of desmin-related cardiomyopathy in hiPSC-derived cardiomyocytes. European Journal of Cell Biology.




